Cybersecurity Training for Engineers
Virtual - Self Paced - Embedded Medical

Pre Market Activities

Regulatory Compliance

Secure Culture

Post Market Activities
Velentium Medical's virtual master class will train you in all aspects of
medical device cybersecurity. The class will train you on the
creation of all artifacts needed for pre-market submission.
The course is tightly integrated across all design and development
disciplines.
FDA Requirements
Detailed instruction around the September 2023 Guidance
EU Requirements
Complying with the EU's MDR regulations
Testing
Fuzz testing, malformed inputs, static analysis (SAST), pen testing, and support for medical device's unique communication mediums
Hardware Overview
Chip selection and attack surfaces including hardware accelerated cryptography
Cryptographic Primitives
Symmetric and asymmetric cryptography inc. AES-128/256, ECC, RSA, HMAC, and CMAC
SBOM
Machine-readable and human-readable software bill of materials (SBOM) - including creation, monitoring, and distributing
Threat Modeling
Asset and process - including system topology, supply chain, manufacturing, deployment, updates, and decommissioning
Governance
Creation of policies, procedures, work instructions as needed for regulatory compliance
Masterclass
(with Certification Exam)
Mastering Embedded Cybersecurity is an in-depth remote-learning course on medical device cybersecurity. It is designed to be completed on-demand and completed at your own pace over an estimated 4 week period.
This course instructs and certifies for competency in embedded cybersecurity and contains assessments required for certifications and continuing education for annual recertification.
Upon enrolling, attendees will receive:
12 months' access to training videos and course content
12 months' access to virtual office hours for live Q&A with the instructor each week (renews with enrollment in continuing education)
12 months' access to a dedicated Slack workspace where attendees and alumni can post and answer questions, read breaking news and network with peers in the embedded cybersecurity community
Upon completion of the program, a professional certification will be granted.
Recommended to be taken over a minimum of 4 weeks. This course instructs and certifies for competence in embedded medical device cybersecurity.
Instructs and certifies for competence in embedded cybersecurity.
Engineers
Developers
Other Technical Roles
Hardware engineers
Systems engineers
Technical managers
Software engineers
Mobile and cloud engineers
Firmware engineers
The course is $2,995 with an optional $595 per year for continuing education to maintain the certification.

$2,995
From a recent student...
"Since I have only been involved with medical device security for a brief time, the Medical Device Cybersecurity Masterclass offered by Velentium Medical was very informative and helpful to understand the landscape of regulatory requirements as well as activities required for medical device manufacturers to consider during the total product development lifecycle.
Currently there are limited training options available on the topic of medical device cybersecurity and this course taught by Christopher Gates is an excellent way to start embracing the subject matter."
Christopher Giudice
Medical Device Cybersecurity Lead
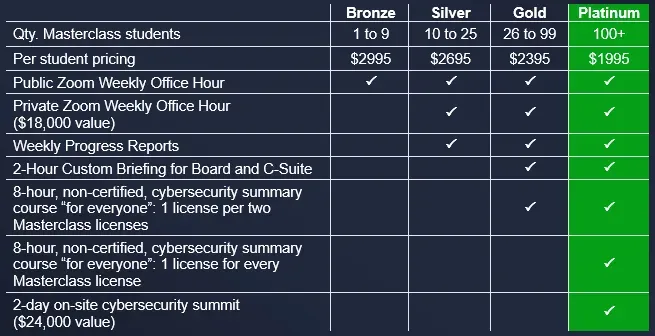
Bundles & Volume Discounts
Need help with budget approval?
Customize this request letter.
On-demand courses address the changes in regulatory compliance and
guidance on secure device design, development, manufacturing, and
postmarket surveillance.
Meet Our Experts
We regularly monitor the latest requirements with FDA and international regulators to assure that the course stays current.

Safe. Secure. Effective.
One stop for secure Medical Device R&D, product development, contract
manufacturing, and postmarket services
Who We Are
© 2025 Velentium, LLC. All Rights Reserved.
