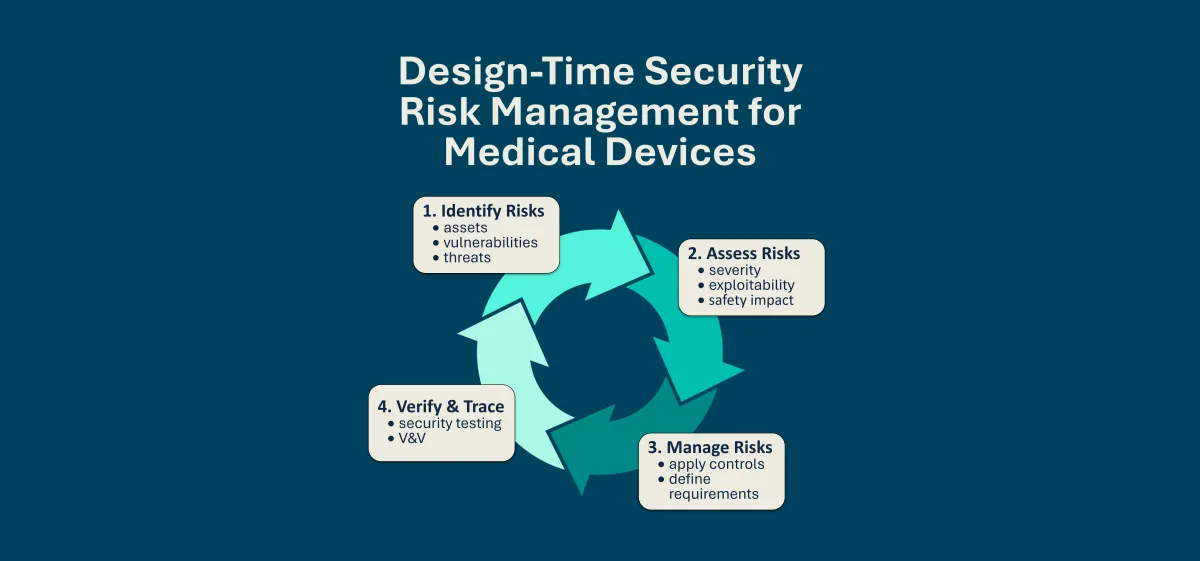
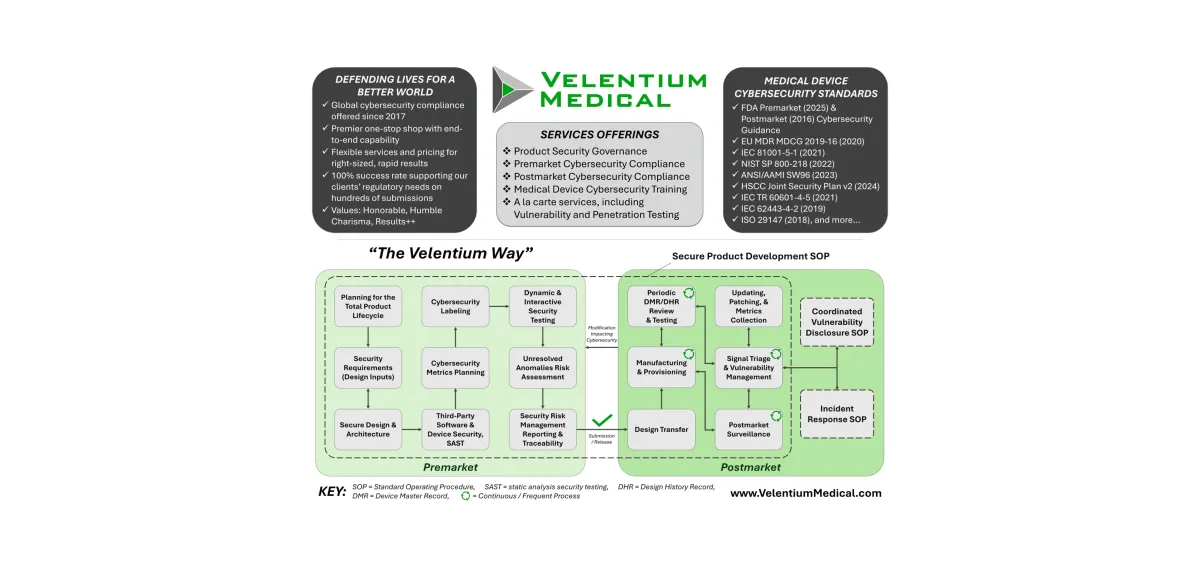
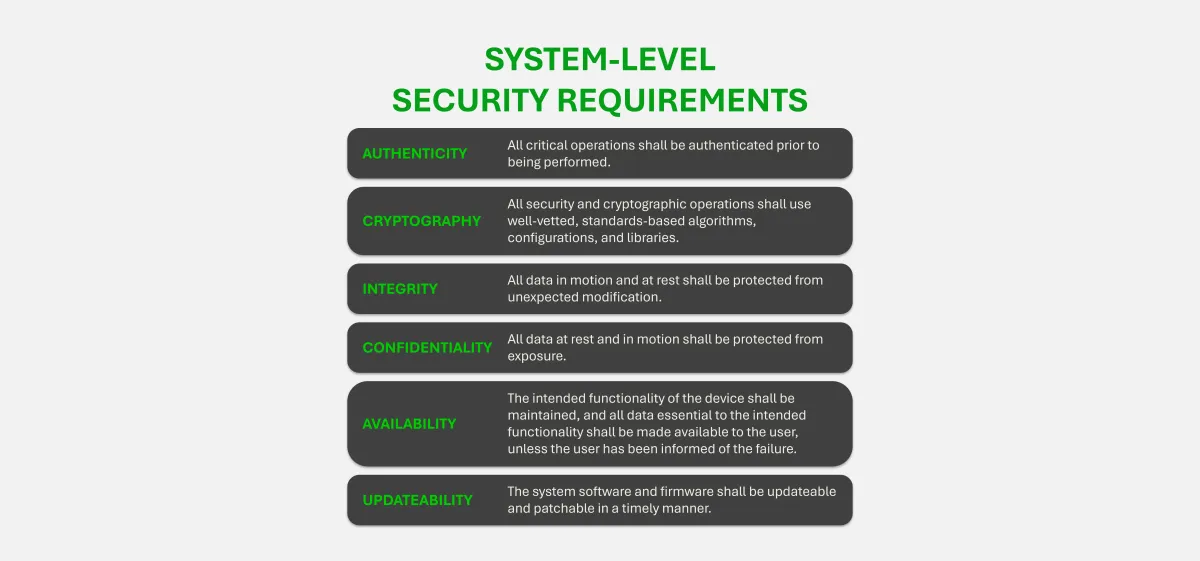
Cybersecurity Labeling for Medical Devices
Cybersecurity labeling connects design intent to real-world device use. It communicates to clinicians, IT administrators, and healthcare delivery organizations how to install, configure, maintain, and retire a device securely.