Total Product Life-Cycle Security for Medical Devices
Download Velentium Medical's Guide to Total Product Life-Cycle Security
Introduction to Velentium Medical’s Cybersecurity Approach
At Velentium Medical, cybersecurity isn’t a checkbox. It’s a commitment to improving and defending lives for a better world. Our Total Product Life-Cycle (TPLC) security process is purpose-built to align with the latest and most rigorous standards from the U.S. FDA, EU MDR/MDCG, IEC 81001-5-1, and more. Whether you're building a brand-new system or sustaining one already in the field, we provide a globally compliant, submission-ready pathway to securing your device from concept to end of support. This guide outlines the phases, activities, and deliverables that make up Velentium Medical’s Secure Product Development Framework (SPDF) for the TPLC. It reflects a proven approach trusted by hundreds of clients and backed by a 100% success rate in regulatory submissions. As a premier one-stop shop for medical device cybersecurity, we flex our services and pricing to meet your needs, whether you need full-service support or expert guidance on specific phases. The table in Section 3.0 maps our security process to the IEC 62304 software development lifecycle. Use it as a practical reference to understand what “secure by design” truly means across every phase of your device’s life.
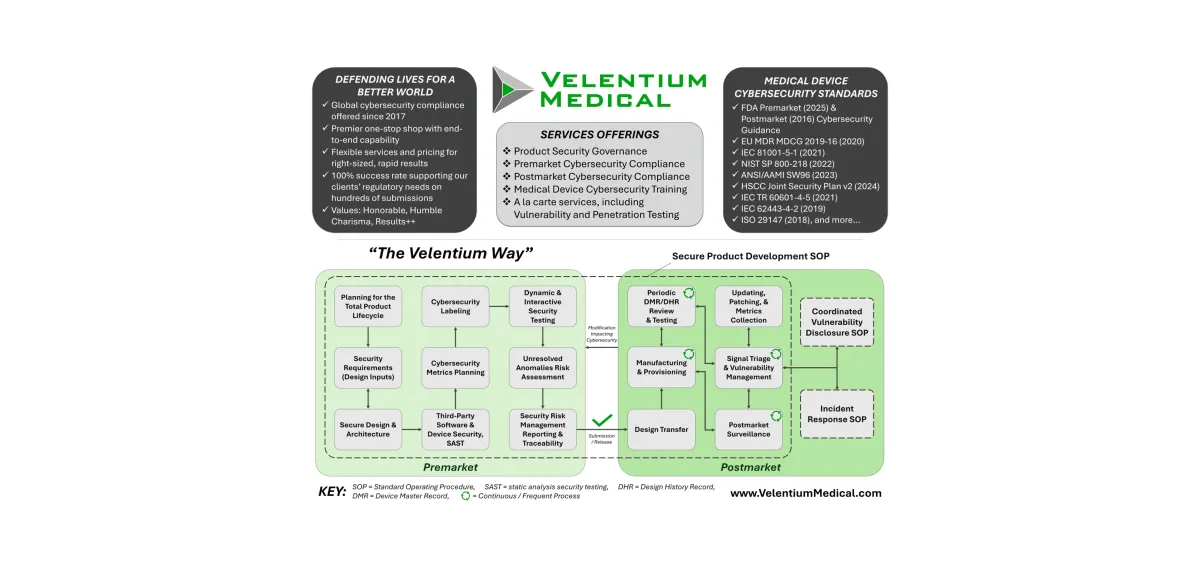
The Velentium Way
The Velentium Medical SPDF includes three Standard Operating Procedures (SOPs):
Secure Product Development SOP – Covers premarket new product development and a majority of postmarket activities, inclusive of surveillance and maintenance. Activities include planning, requirements derivation and analysis, threat modeling, cybersecurity risk assessment, architecture and controls reporting, Software Bill of Materials (SBOMs), labeling, metrics, security testing, security assessments of software bugs, traceability, and more.
Coordinated Vulnerability Disclosure SOP – Covers coordinated vulnerability disclosure during postmarket, beginning with the intake of security reports from external parties and ending with disclosure to customers, government agencies, and other stakeholders.
Incident Response SOP – Covers the response, recovery, and recording of security events deemed to be true security incidents happening during postmarket.
Velentium Medical offers access to these SOP templates and templates for regulatory artifacts, plus consulting and support for manufacturers so they can implement procedures in their QMS, train their personnel on operating within the procedures, develop safe and secure products, and create globally compliant DHF and DMR/DHR items.
The graphic below shows this holistic process in a simplified flow diagram, although the actual implementation may vary slightly per project and organization.

The PDF version of SEC-GUIDE-07 Total Product Lifecycle Security contains a table aligning these security activities with IEC 62304 lifecycle phases and provides a discussion of the outcomes and deliverables of each security activity.
