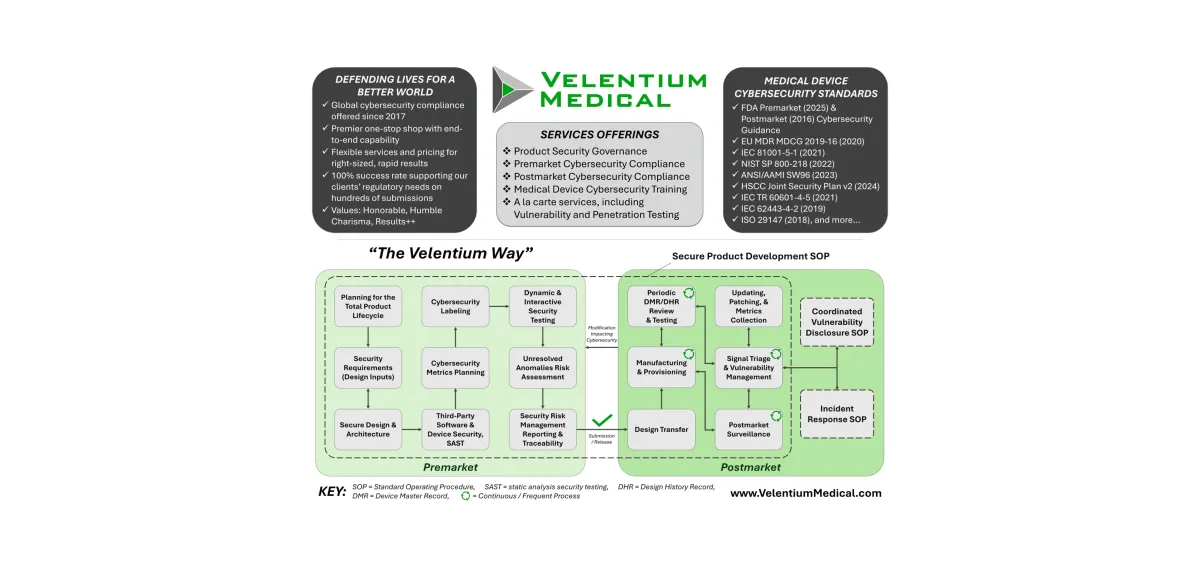
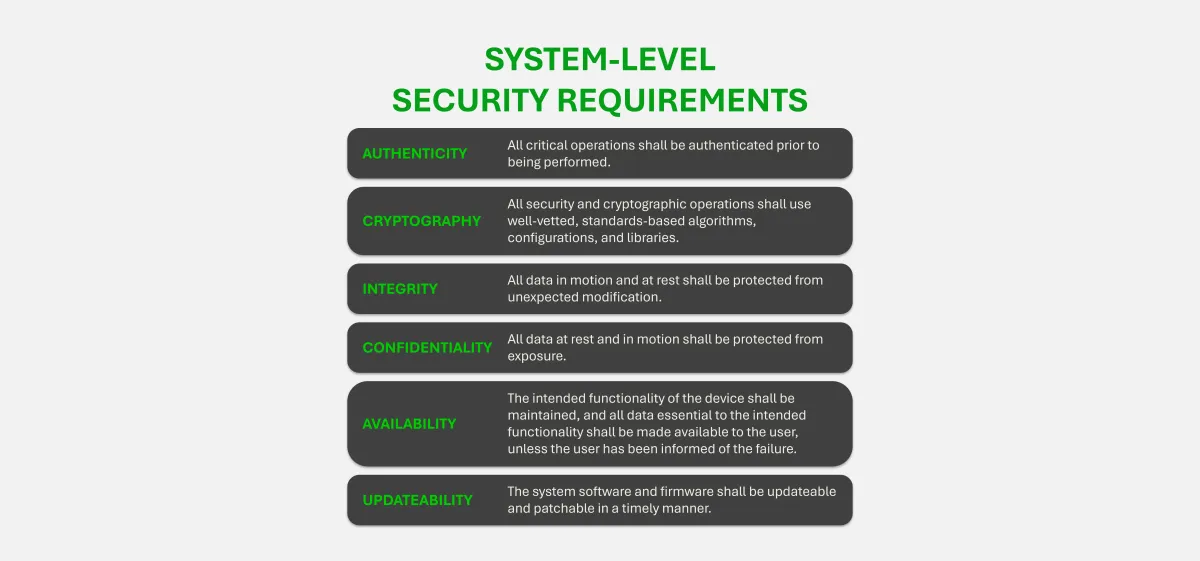
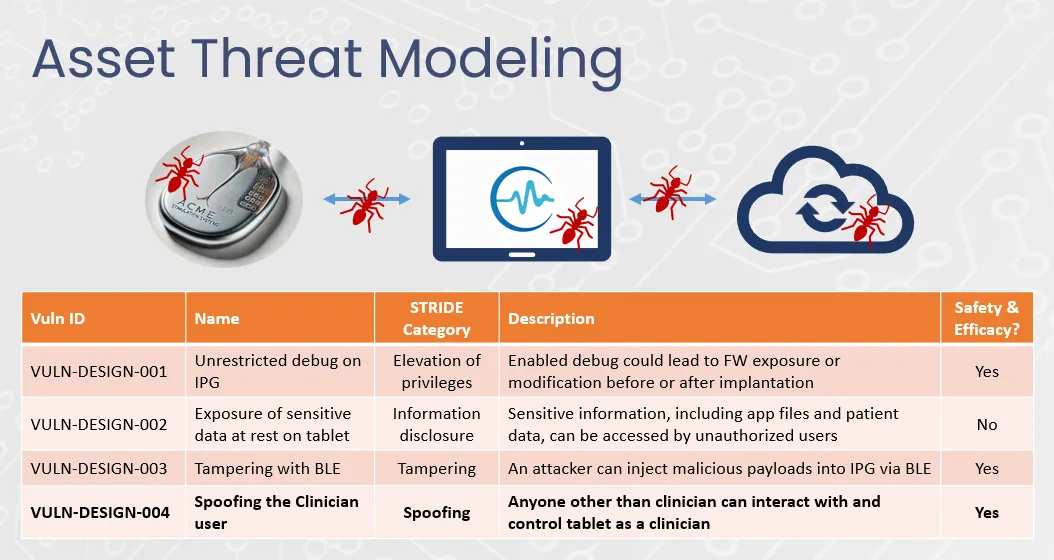
Total Product Life-Cycle Security for Medical Devices
Cybersecurity isn’t just a test before launch. At Velentium Medical, it’s built into every phase of development and maintenance. Our Total Product Life-Cycle process ensures your device is secure by design and safe in the field, backed by our templates and expertise to help you navigate the full process.